
发展有机小分子来调控疾病相关的靶标蛋白
Subtopic III: Discover and develop small-molecule organic compounds which effectively regulate the functions of disease-relevant biological target molecules.
Development of Small-Molecule Inhibitors of Bcl-2 Family Proteins
Xun Li, Bingcheng Zhou, Yan Li, Xinglong Zhang, Zhimin Shi, Cuixia Zhu, Wenwen Li and Renxiao Wang*
State Key Laboratory of Bioorganic Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 354 Fenglin Road, Shanghai 200032, China, P. R,
E-mail: wangrx@mail.sioc.ac.cn
As key regulators of cell apoptosis, Bcl-2 family proteins (including Bcl-2, Bcl-xL, Mcl-1, etc.) have drawn much attention as potential drug targets since late 1990s. Oligonucleotide antisenses and small-molecule inhibitors targeting at these anti-apoptotic proteins are being tested in clinical trials as anti-cancer therapies [1-3]. Recently, Bcl-2 family proteins are found to play important roles in other cellular events. For example, Bcl-2 was revealed to inhibit autophagy via an interaction with Beclin-1[4]; Bcl-xL and Bcl-2 can inhibit cell proliferation besides inhibiting apoptosis [5]. These interesting findings have promoted Bcl-2 family proteins as promising drug targets even further.
As a research group affiliated to the State Key Laboratory of Bioorganic Chemistry, we have been working on discovering and developing small-molecule inhibitors targeting at the Bcl-2 family proteins since 2006. The basic flowchart of our work can be summarized as following:
(1) Lead discovery: Our group is equipped with high-performance computer hardware and a panel of main-stream software for molecular modeling. With our expertise in computer-aided drug design, we have conducted structure-based virtual screening of over one million organic molecules against Bcl-2 family proteins. Samples of top-ranked candidates were purchased for subsequent testing.
(2) In vitro binding assay: Obtained compounds were firstly tested in in vitro binding assay. For this purpose, we have successfully expressed and purified the target proteins (Bcl-2, Bcl-xL, and Mcl-1), and have set up a fluorescence polarization (FP) binding assay and a surface plasmon resonance (SPR) binding assay. Both binding assays were employed to obtain more reliable results.
(3) Tumor cell lines: Active compound identified in the in vitro binding assays were then tested in MTT assays on several tumor cell lines that have high-level expression of Bcl-2 family proteins. Compounds that could effectively inhibit the growth of tumor cells were further studied with respect to their mechanisms in the regulation of apoptosis.
(4) Lead optimization: Some compounds which exhibited promising potency in both in vitro binding assay and tumor cell lines were selected as lead compounds for further developments. Molecular modeling of the binding modes of these compounds to the target protein suggested structural optimizations. Derivatives of the lead compounds were synthesized accordingly and then tested in bioassays.
(5) Structure determination: Through collaborations with other groups, crystals of the complexes formed between Bcl-2 family proteins and several selected compounds are currently under development. Crystal structure of the Bcl-xL protein in apo-form has already been obtained in a reasonable resolution. Crystals of the complexes formed between Bcl-2 family proteins and some selected lead compounds are currently under development. NMR measurements (such as HSQC) were also employed to characterize the interactions between our compounds with the target protein.
We have tested over 500 compounds from virtual screening so far. Four classes of lead compounds showing micro-molar inhibitory activities to Bcl-xL were discovered. One example is shown in Figure 1. Patent petitions regarding these compounds have been filed recently. We are now optimizing the chemical structures of these lead compounds to further improve their biological potencies. Some 150 derivatives of these lead compounds have already been synthesized and tested. Lead optimizations will carry on until some compounds potent enough for pre-clinical trials are obtained.
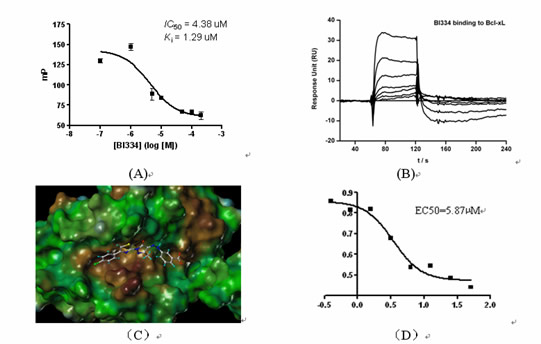
Figure 1. (A) In vitro binding of compound BI334 to Bcl-xL measured in an FP-based binding assay; (B) In vitro binding of compound BI334 to Bcl-xL measured in an SPR-based binding assay; (C) Predicted binding mode of compound BI334 to Bcl-xL; (D) Growth inhibition on breast cancer cell line MDA- MB-231 demonstrated by BI334.
In summary, we are able to combine our in-depth expertise in computer-aided design with organic synthesis and biological assays to discover and develop novel small-molecule inhibitors of Bcl-2 family proteins. Indeed we have obtained some promising compounds in a relatively short period of time. The same cost-effective approach of course can be applied to other biological targets as well. The multi-disciplinary infrastructure of the State Key Laboratory of Bioorganic Chemistry also provides an ideal external environment for our work so that some in-depth research can be conducted through collaborations with other groups if such research is beyond our own capabilities.
References
1. Jansen, B.; Schlagbauer-Wadl, H.; Brown, B. D.; Bryan, R. N.; van Elsas, A.; Muller, M.; Wolff, K.; Eichler, H. G.; Pehamberger, H. bcl-2 antisense therapy chemosensitizes human melanoma in SCID mice. Nature Med. 1998, 4, 232-234.
2. Oltersdorf, T.; Elmore, S. W.; Shoemaker, A. R.; Armstrong, R. C.; Augeri, D. J.; Belli, B. A.; Bruncko, M.; Deckwerth, T. L.; Dinges, J.; Hajduk, P. J.; Joseph, M. K.; Kitada, S.; Korsmeyer, S. J.; Kunzer, A. R.; Letai, A.; Li, C.; Mitten, M. J.; Nettesheim, D. G.; Ng, S.; Nimmer, P. M.; O'Connor, J. M.; Oleksijew, A.; Petros, A. M.; Reed, J. C.; Shen, W.; Tahir, S. K.; Thompson, C. B.; Tomaselli, K. J.; Wang, B. L.; Wendt, M. D.; Zhang, H. C.; Fesik, S. W.; Rosenberg, S. H. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature 2005, 435, 677-681.
3. Nguyen, M.; Marcellus, R. C.; Roulston, A.; Watson, M.; Serfass, L.; Madiraju, S. R. M.; Goulet, D.; Viallet, J.; Belec, L.; Billot, X.; Acoca, S.; Purisima, E.; Wiegmans, A.; Cluse, L.; Johnstone, R. W.; Beauparlant, P.; Shore, G. C. Small molecule obatoclax (GX15-070) antagonizes MCL-1 and overcomes MCL-1-mediated resistance to apoptosis. Proc. Natl. Acad. Sci. USA 2007, 104, 19512-19517.
4. Pattingre, S.; Tassa, A.; Qu, X. P.; Garuti, R.; Liang, X. H.; Mizushima, N.; Packer, M.; Schneider, M. D.; Levine, B. Bcl-2 antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell 2005, 122, 927-939.
5. Janumyan, Y. M.; Sansam, C. G.; Chattopadhyay, A.; Cheng, N. L.; Soucie, E. L.; Penn, L. Z.; Andrews, D.; Knudson, C. M.; Yang, E. Bcl-xL/Bcl-2 coordinately regulates apoptosis, cell cycle arrest and cell cycle entry. EMBO J. 2003, 22, 5459-5470.
Copyright ©2007-2021 上海盈赛思信息科技有限公司 网站备案号:沪ICP备2021015625号-2 ![]() 沪公网安备:正在申请中
沪公网安备:正在申请中
Technical Support(技术支持): yingsaisi@foxmail.com
